Background: Patients with acute myeloid leukemia (AML) who are older or ineligible for intensive induction chemotherapy have limited treatment options and poor survival. In the VIALE-A study, venetoclax (Ven) + azacitidine (Aza) improved overall survival and response rates compared with placebo (Pbo) + Aza in older or unfit patients with newly diagnosed AML (DiNardo et al. N Engl J Med. 2020). Although cytopenia is common in AML, Ven+Aza was associated with hematologic adverse events in 83% of patients in the VIALE-A study (vs 69% in the Pbo+Aza arm). Here, the frequency and management of cytopenia are analyzed in patients achieving a best response of complete remission (CR) or CR with partial hematologic recovery (CRh) in the VIALE-A study.
Methods: This double-blind, Pbo-controlled, multicenter Phase 3 study (NCT02993523) enrolled patients with newly diagnosed AML who were ineligible for intensive chemotherapy due to age ≥75 years or comorbidities. Patients were randomized 2:1 to receive 75 mg/m2 Aza (Days 1-7 of each 28-day cycle) plus either daily 400 mg Ven (Ven+Aza) or Pbo (Pbo+Aza). Disease response was assessed via bone marrow aspirate and biopsy at the end of Cycle 1 and at least every 3 cycles thereafter. After patients achieved blast clearance (bone marrow blasts <5%), various dosing modifications were implemented to manage cytopenia (Table 1). Cytopenia, defined here as Grade 4 neutropenia (absolute neutrophil count <500/μL) or Grade 4 thrombocytopenia (platelet count <25×103/μL) lasting ≥7 days, was assessed using laboratory data.
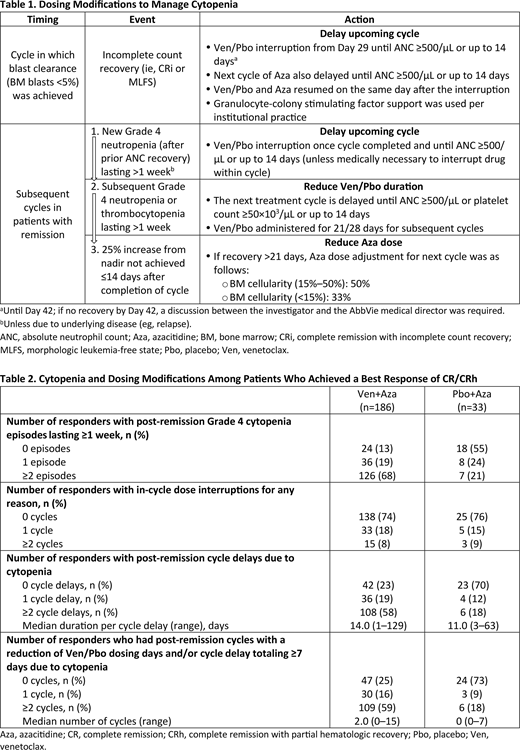
Results: In total, 186 of 283 (66%) Ven+Aza-treated patients and 33 of 144 (23%) Pbo+Aza-treated patients achieved a best response of CR or CRh (CR/CRh). Of patients with a best response of CR/CRh in the Ven+Aza arm, 77% achieved blast clearance by the end of Cycle 1, 88% by the end of Cycle 2, 92% by the end of Cycle 3, and 98% by the end of Cycle 4. Of patients with a best response of CR/CRh in the Pbo+Aza arm, 33% achieved blast clearance by the end of Cycle 1, 55% by the end of Cycle 2, 76% by the end of Cycle 3, and 91% by the end of Cycle 4. After achieving blast clearance, 75% and 67% of patients in the Ven+Aza and Pbo+Aza arms, respectively, had a delay of the next cycle, with a median duration per cycle delay post-blast clearance (range) of 9.0 (1-39) and 5.5 (1-21) days.
Among CR/CRh patients, more patients receiving Ven+Aza versus Pbo+Aza had post-remission cytopenia (87% vs 45%; Table 2). A similar percentage of CR/CRh patients in both arms (Ven+Aza, 26%; Pbo+Aza, 24%) had in-cycle dose interruptions (ie, days without Ven/Pbo exposure between a cycle's first and last Ven/Pbo dose), with a median duration (range) of 2.0 days (1-20) for Ven+Aza and 1.0 (1-13) for Pbo+Aza. A greater proportion of CR/CRh patients experienced post-remission cycle delays due to cytopenia in the Ven+Aza arm (77%) than in the Pbo+Aza arm (30%). Furthermore, a higher percentage of CR/CRh patients had post-remission cycles with a reduction in Ven/Pbo dosing days and/or cycle delays totaling ≥7 days due to cytopenia in the Ven+Aza arm (75%) than in the Pbo+Aza arm (27%). Among these patients, the median percentage of days without Ven/Pbo administration while in remission (out of the total number of days in remission) was 18% (range, 1-69) in the Ven+Aza arm and 13% (3-44) in the Pbo+Aza arm. Ultimately, 129 CR/CRh patients (69%) in the Ven+Aza arm and 10 (30%) in the Pbo+Aza arm received ≤21-day Ven/Pbo dosing post-remission, with a median time from remission to first ≤21-day cycle (range) of 92.0 days (1-480) for Ven+Aza and 74.0 days (6-405) for Pbo+Aza.
Conclusion: In the VIALE-A study of older or unfit patients with newly diagnosed AML, the majority of responding patients in the Ven+Aza arm required dosing modifications to manage cytopenia, of which delays between cycles or within-cycle reductions of Ven dosing days were most common. Post-remission cytopenia and dosing modifications were more frequent with Ven+Aza versus Pbo+Aza treatment. The impact of cytopenia and dosing modifications on patient outcomes with Ven+Aza is currently being analyzed and will be reported at a future date.
Pratz:AbbVie: Other: Scientific Advisory Board, Research Funding; Boston BioMedical: Consultancy; Astellas: Other: Scientific Advisory Board, Research Funding; Jazz Pharmaceutical: Consultancy; Millennium: Research Funding; Daiichi Sankyo: Research Funding; Agios: Other: Scientific Advisory Board, Research Funding; Celgene: Other: Scientific Advisory Board. DiNardo:MedImmune: Honoraria; Agios: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Jazz: Honoraria; Takeda: Honoraria; AbbVie: Consultancy, Honoraria, Research Funding; Syros: Honoraria; Notable Labs: Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; ImmuneOnc: Honoraria; Novartis: Consultancy; Calithera: Research Funding. Selleslag:Amgen: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Astellas: Consultancy, Honoraria, Speakers Bureau; Takeda: Consultancy, Honoraria, Speakers Bureau; Janssen Cilag: Consultancy, Honoraria, Speakers Bureau; AbbVie: Consultancy, Honoraria, Speakers Bureau; Belgian College: Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Speakers Bureau; MSD: Consultancy, Honoraria, Speakers Bureau; Incyte: Consultancy, Honoraria; Alexion: Consultancy, Honoraria; GSK: Consultancy, Honoraria; Teva: Consultancy, Honoraria, Speakers Bureau; Sanofi: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Speakers Bureau. Yamamoto:AbbVie: Consultancy, Honoraria, Research Funding; Astra-Zeneca: Consultancy, Research Funding; Zenyaku: Research Funding; Sumitomo Dainippon: Honoraria; Sanofi: Honoraria; Pfizer: Honoraria; Otsuka: Consultancy, Honoraria, Research Funding; Ono: Consultancy, Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Nippon Shinyaku: Honoraria, Research Funding; Mundipharma: Consultancy, Honoraria, Research Funding; MSD: Consultancy, Honoraria, Research Funding; Mochida: Honoraria; Meiji Seika Pharma: Consultancy, Honoraria; Kyowa Kirin: Honoraria; Janssen: Honoraria; Gilead Sciences: Research Funding; IQIVA/Incyte: Research Funding; HUYA: Consultancy; IQIVA/HUYA: Honoraria; Daiichi Sankyo: Consultancy; Eisai: Consultancy, Honoraria, Research Funding; Chugai: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Yakult: Research Funding; Stemline Therapeutics: Consultancy; Solasia Pharma: Research Funding; SymBio: Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria; Bayer: Research Funding; Aichi Cancer Center: Current Employment. Konopleva:AbbVie: Consultancy, Research Funding; Cellectis: Research Funding; Agios: Research Funding; Sanofi: Research Funding; Amgen: Consultancy; Reata Pharmaceutical Inc.;: Patents & Royalties: patents and royalties with patent US 7,795,305 B2 on CDDO-compounds and combination therapies, licensed to Reata Pharmaceutical; Ascentage: Research Funding; Eli Lilly: Research Funding; Stemline Therapeutics: Consultancy, Research Funding; Calithera: Research Funding; Forty-Seven: Consultancy, Research Funding; Ablynx: Research Funding; Rafael Pharmaceutical: Research Funding; F. Hoffmann La-Roche: Consultancy, Research Funding; Kisoji: Consultancy; AstraZeneca: Research Funding; Genentech: Consultancy, Research Funding. McDonald:venetoclax advisory board in South Africa (in CLL context): Consultancy; Alberts Cellular Therapy: Current Employment. Babu:Genentech, Inc./ F. Hoffmann-La Roche: Research Funding; Novartis: Research Funding; Janssen Oncology: Research Funding; Syndax: Research Funding; Nektar: Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding; Alexion Pharmaceuticals: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding, Speakers Bureau; Lilly: Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding; Bayer: Honoraria; AstraZeneca: Consultancy, Honoraria; AstraZeneca/MedImmune: Research Funding; Argenx: Consultancy, Research Funding; Boehringer Ingelheim: Consultancy; Merck: Research Funding; AbbVie: Research Funding; TG Therapeutics: Research Funding; Amgen: Research Funding; Lutheran Hospital: Other; Fort Wayne Medical Oncology & Hematology: Current Employment, Current equity holder in publicly-traded company; Sanofi: Research Funding. Stevens:Amgen, MorphoSys: Consultancy. Kantarjian:BioAscend: Honoraria; Delta Fly: Honoraria; Abbvie: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Ascentage: Research Funding; Janssen: Honoraria; Adaptive biotechnologies: Honoraria; Aptitute Health: Honoraria; Oxford Biomedical: Honoraria; Daiichi-Sankyo: Honoraria, Research Funding; BMS: Research Funding; Jazz: Research Funding; Immunogen: Research Funding; Novartis: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Sanofi: Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees. Traina:University of São Paulo at Ribeirão Preto Medical School: Current Employment; AbbVie: Other: Principal Investigator for Protocol Number M15-656 . Venditti:Jazz: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); AbbVie: Consultancy, Honoraria, Other: TRAVEL, ACCOMODATIONS, EXPENSES (paid by any for-profit health care company); Janssen: Consultancy, Honoraria, Other: TRAVEL, ACCOMODATIONS, EXPENSES (paid by any for-profit health care company); Amgen: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); Pfizer: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Speakers Bureau; Novartis: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company). Mayer:AbbVie: Research Funding; Principia Biopharma: Research Funding. Montez:F. Hoffmann-La Roche: Current equity holder in publicly-traded company; Genentech, Inc.: Current Employment. Ramsingh:Roche: Current equity holder in publicly-traded company, Ended employment in the past 24 months; Genentech: Current Employment, Current equity holder in publicly-traded company. Jin:Genentech: Current Employment. Ainsworth:AbbVie: Current Employment, Current equity holder in publicly-traded company. Duan:AbbVie: Current Employment, Other: may hold stock or options. Svensson:AbbVie: Current Employment, Current equity holder in publicly-traded company. Werner:AbbVie: Current Employment, Current equity holder in publicly-traded company. Potluri:AbbVie: Current Employment, Other: may hold stock or stock options. Jonas:AbbVie: Consultancy, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding; Amgen: Consultancy, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding; GlycoMimetics: Consultancy, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding; Celgene: Consultancy, Research Funding; Jazz: Consultancy, Research Funding; Takeda: Consultancy; Tolero: Consultancy; Treadwell: Consultancy; Forty Seven: Research Funding; Accelerated Medical Diagnostics: Research Funding; AROG: Research Funding; Daiichi Sankyo: Research Funding; F. Hoffmann-La Roche: Research Funding; Forma: Research Funding; Genentech/Roche: Research Funding; Hanmi: Research Funding; Incyte: Research Funding; LP Therapeutics: Research Funding; Pfizer: Research Funding; Pharmacyclics: Research Funding; Sigma Tau: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal